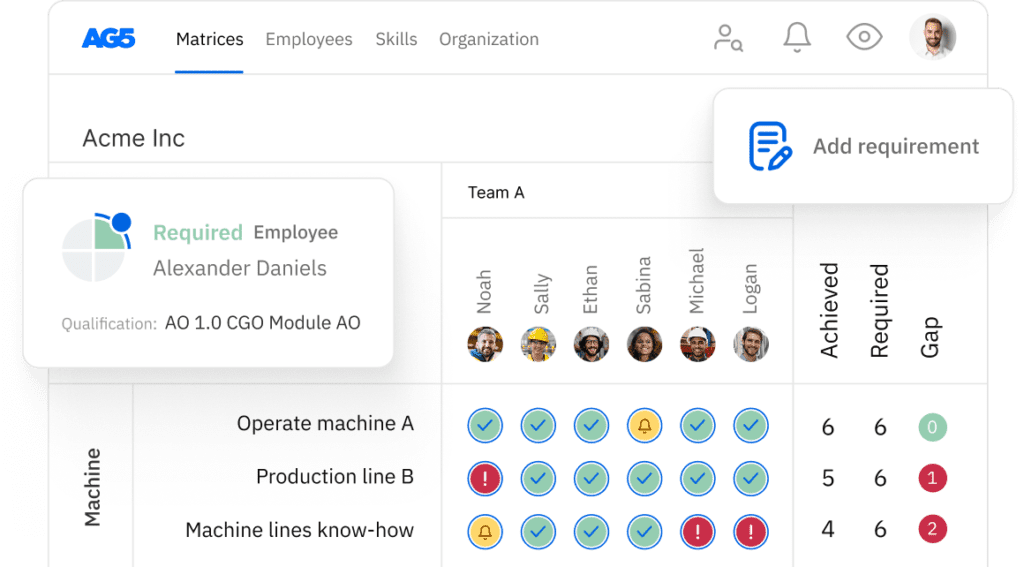
FDA regulations skills matrix template
A skills matrix template is a tool teams can use to effectively manage and assess their FDA regulations skills and knowledge.
Download your free template here
Overview Copied
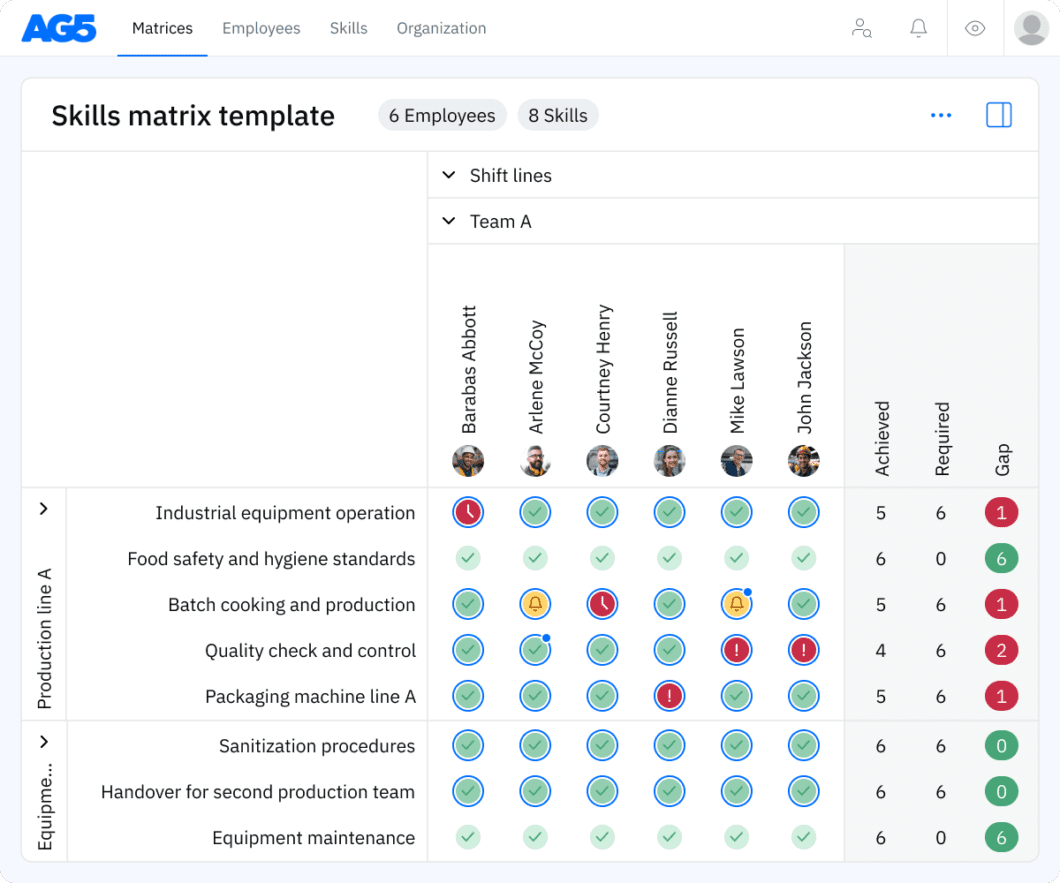
A skills matrix template is a tool that organizations can use to effectively manage and assess the skills and certification statuses of individual employees or teams.
For example, you can use this FDA regulations skills matrix to pinpoint employees’ specific competencies and certification statuses in areas such as regulatory affairs, document control, and quality management systems for medical manufacturing.
Regulatory Knowledge and Documentation
- Regulatory Affairs Knowledge
- Document Control
- Quality Management Systems (QMS)
- Regulatory Affairs Certification (RAC)
- Certified Manager of Quality/Organizational Excellence (CMQ/OE)
- Good Clinical Practices (GCP)
Compliance Processes and Management
- Risk Management
- Auditing
- Change Control
- SOP Writing
- Training and Education
- Certified in Risk and Information Systems Control (CRISC)
- Certified Internal Auditor (CIA)
- Certified Change Management Professional (CCMP)
Product Safety and Reporting
- Complaint Handling
- Adverse Event Reporting
- Data Integrity
- Labeling Compliance
- Post-Market Surveillance
- Recall Procedures
- Good Manufacturing Practices (GMP)
- Certified Safety Professional (CSP)
- Certified in Healthcare Privacy Compliance (CHPC)
System and Process Implementation
- Serialization
- Electronic Records and Signatures
- Supplier Management
- Continual Improvement
- Serialization Professional Certification (SPC)
- Certified Records Manager (CRM)
- Certified Supplier Quality Professional (CSQP)
Benefits Copied
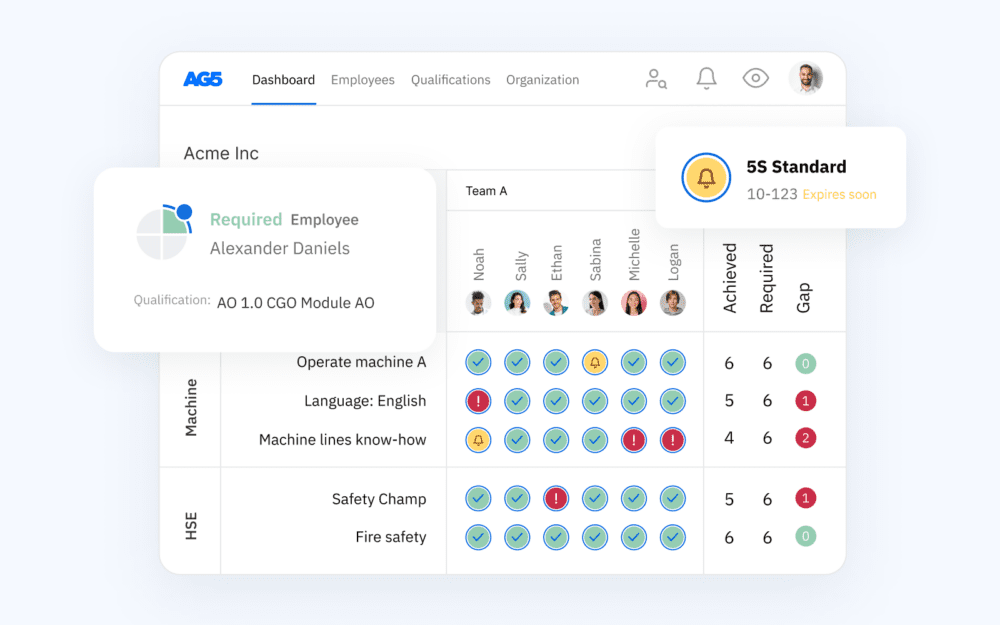
Using a FDA regulations skills matrix can benefit your teams in various ways – especially if used with AG5’s skills management software. Our platform has several features built specifically to meet FDA requirements. They include:
- Audit trails. We automatically timestamp and document the history of all data changed – so you can meet the FDA requirements for accurate record-keeping and data traceability.
- Data management approvals. Establish multi-person workflow approvals for confirming and updating production records – so you can meet FDA requirements for data accuracy and change management.
- Data authorization. Set up two-factor authentication for approvals and data updates to ensure data integrity and enhanced access controls.
Download the free Excel FDA regulations skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
Customizing a skills matrix Copied
The scope of a skills matrix can be as large or small as you choose. For example, it could cover your entire organization – or only those teams or individuals with product safety-oriented roles.
To customize a skills matrix to your needs, you should:
- Define focus. Clarify if the skills matrix pertains to an entire department or specific roles within it.
- Identify key skills. List essential competencies for each role or area of operation.
- Establish proficiency levels. Define skill proficiency levels, aligning them with job requirements.
- Set evaluation criteria. Create a clear evaluation process, considering on-the-job performance and training qualifications.
- Regularly review and update. Ensure regular reviews to adapt to evolving roles, technologies, and organizational goals.
Certifications for FDA regulations Copied
- Certified Safety Professional (CSP)
- Regulatory Affairs Certification (RAC)
- Certified Manager of Quality/Organizational Excellence (CMQ/OE)
- Certified in Risk and Information Systems Control (CRISC)
- Certified Internal Auditor (CIA)
- Certified Change Management Professional (CCMP)
- Certified in Healthcare Privacy Compliance (CHPC)
- Certified Records Manager (CRM)
- Certified Supplier Quality Professional CSQP
Looking for alternatives to Excel for your skills matrix templates? Copied
AG5’s skills management software enables you to visualize and close skills gaps across your organization – no need for complicated Excel spreadsheets. You’ll use intuitive skills matrices that bring together skills data and job requirements – and are always up to date.
Frequently asked question Copied
-
What are the benefits of using an FDA regulations skills matrix?
-
How do I use an FDA regulations skills matrix?
-
What if I want to take my skills management to the next level?
Author Copied
Revisions Copied
Written by: Rick van Echtelt
Copy edited by: Adam Kohut
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management