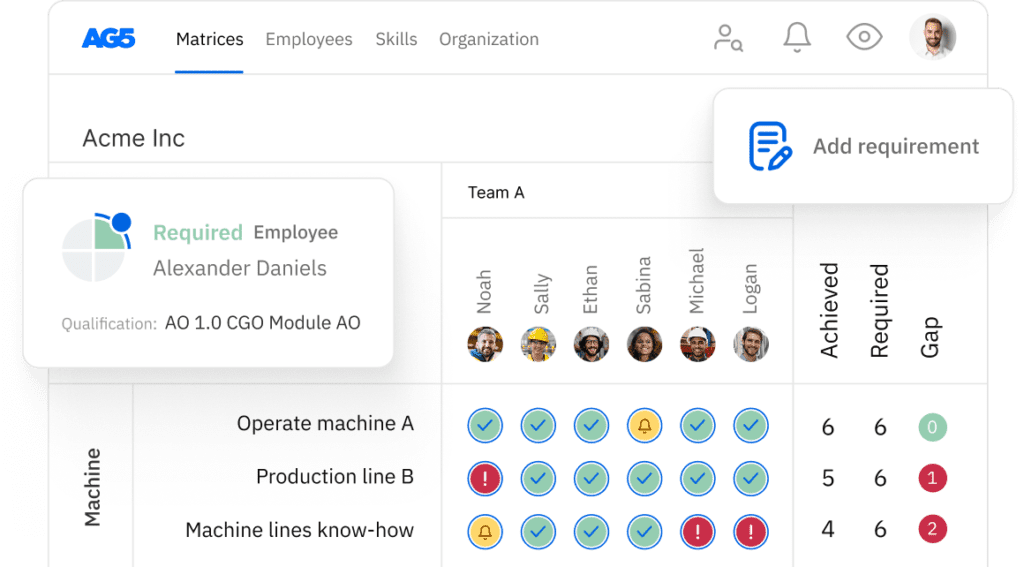
ISO 13485 skills matrix template
A skills matrix template is a tool teams can use to effectively manage and assess their ISO 13485 skills and knowledge.
Download your free template here
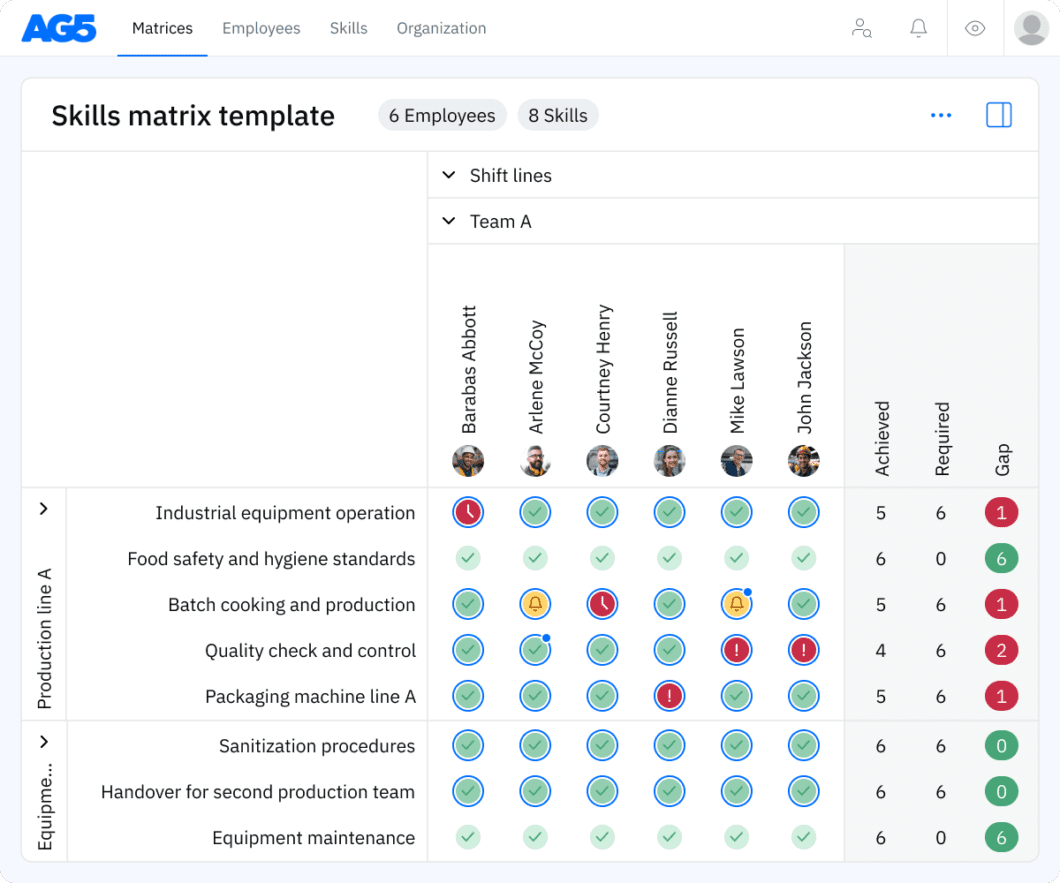
Overview Copied
With our free ISO 13485 skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
ISO 13485 Quality Management System (QMS)
- Understanding of ISO 13485 requirements
- Development and maintenance of QMS for medical devices
- Document control and record-keeping per ISO 13485
- Implementation of risk management processes (e.g., ISO 14971)
- Managing and maintaining quality manuals and SOPs
- Conducting internal audits according to ISO 13485
- Implementation of corrective and preventive actions (CAPA)
- Change control management within QMS
Regulatory compliance
- Compliance with FDA 21 CFR Part 820, EU MDR, or other relevant regulations
- Understanding of global regulatory requirements for medical devices
- Ensuring compliance with product lifecycle management
- Compliance with medical device reporting requirements
- Managing external regulatory audits and inspections
- Certification and recertification processes for ISO 13485
Design and development
- Design control processes (e.g., design history files, design review)
- Verification and validation of medical devices
- Risk management during design and development
- Documenting design inputs, outputs, and changes
- Integration of usability and human factors engineering
- Conducting design failure mode and effects analysis (DFMEA)
Production and process controls
- Process validation (e.g., IQ/OQ/PQ)
- Control of production processes and work environments
- Traceability of materials and components
- Managing non-conforming products
- Inspection, measuring, and test equipment calibration
- Cleanroom environment control and monitoring
Supplier quality management
- Supplier qualification and monitoring
- Supplier audits and performance evaluation
- Managing supplier corrective actions
- Supplier compliance with ISO 13485 and related standards
- Establishing and maintaining supplier agreements
Training and competency management
- Ensuring personnel competency in ISO 13485 requirements
- Conducting and documenting training for relevant personnel
- Maintenance of training records
- Evaluating training effectiveness
- Ongoing professional development within QMS roles
Benefits Copied
In the context of ISO standards such as ISO 13485, ISO 9001 ISO 17025, ISO 14971, ISO 45001 and ISO 14001 certification, skills management software helps organizations manage and track training records, personnel certifications and competencies required for compliance with these standards.
Download the free Excel ISO 13485 skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management