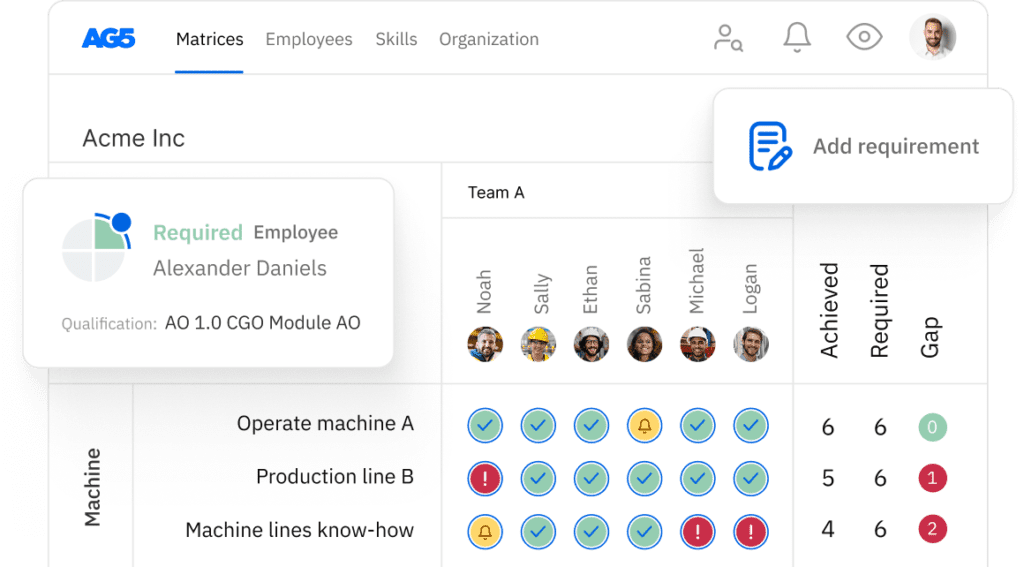
Biocompatibility testing skills matrix template
A skills matrix template is a tool teams can use to effectively manage and assess their biocompatibility testing skills and knowledge.
Download your free template here
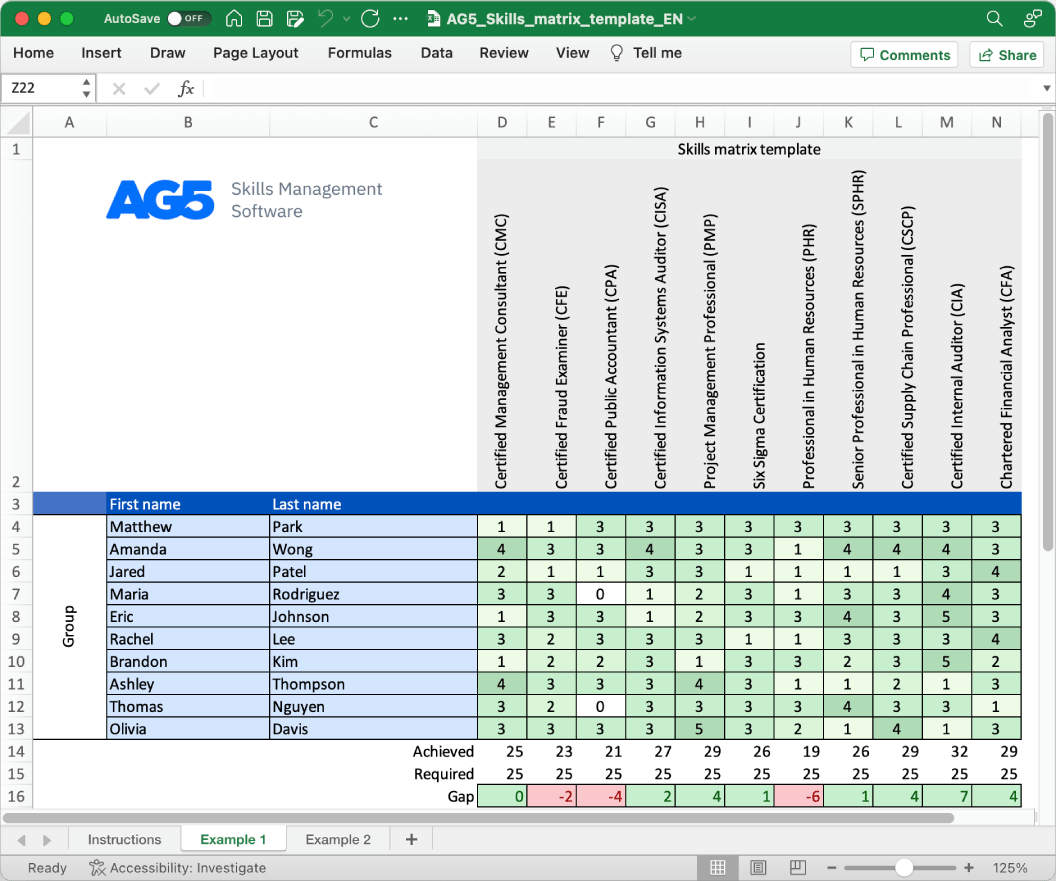
Overview Copied
With our free biocompatibility skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
- Certified Biocompatibility Specialist
- ISO 10993 Certified Professional
- Biocompatibility Testing Certification
- ASTM F748 Biocompatibility Certification
- Medical Device Biocompatibility Certification
- Biological Evaluation of Medical Devices Certification
- FDA Biocompatibility Compliance Certification
- ISO 14971 Risk Management Certification
- Biocompatibility Regulatory Affairs Certification
- ISO 10993-1 Biocompatibility Expert
- Certified Biocompatibility Engineer
- ISO 17025 Accreditation for Biocompatibility Testing
- Biological Safety Officer (BSO) Certification
- ISO 9001 Certification for Biocompatibility Testing
- Certified Biocompatibility Auditor
- Certified ISO 13485 Lead Auditor
- European Notified Body Certification for Biocompatibility
- ANSI/AAMI/ISO 11137 Sterilization Validation Certification
- FDA 21 CFR Part 11 Compliance Certification
- Certified Medical Device Risk Manager
Benefits Copied
Skills management software is important in biocompatibility to effectively track and manage the specialized skills and qualifications of personnel involved in biocompatibility testing and evaluation. It ensures compliance with industry standards, facilitates skill matching, enables efficient resource allocation, and supports effective decision-making in biocompatibility projects and initiatives.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management