Quality by design (QbD) skills matrix template
A skills matrix template is a tool teams can use to effectively manage and assess their QbD skills and knowledge.
Download your free template here
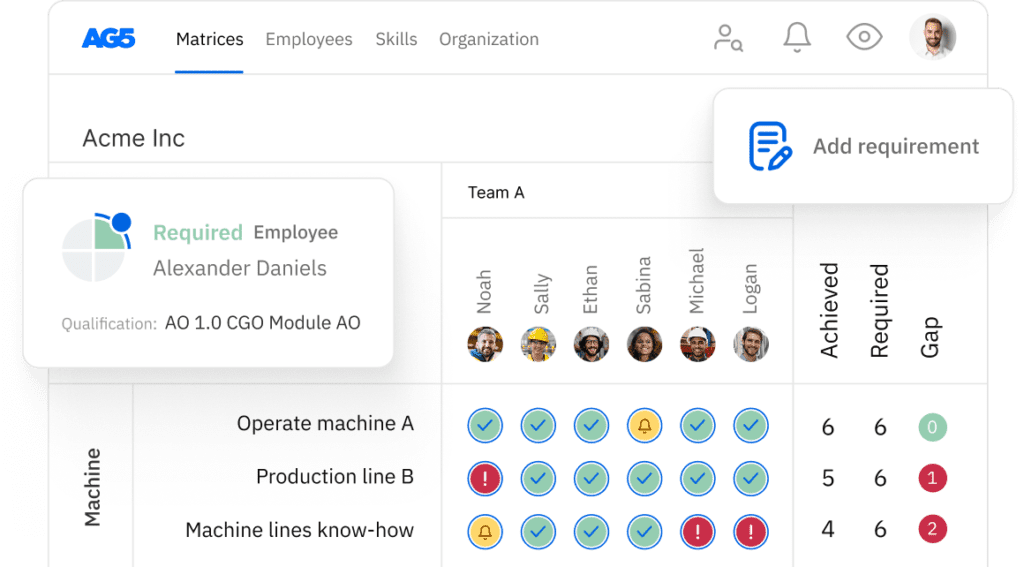
Overview Copied
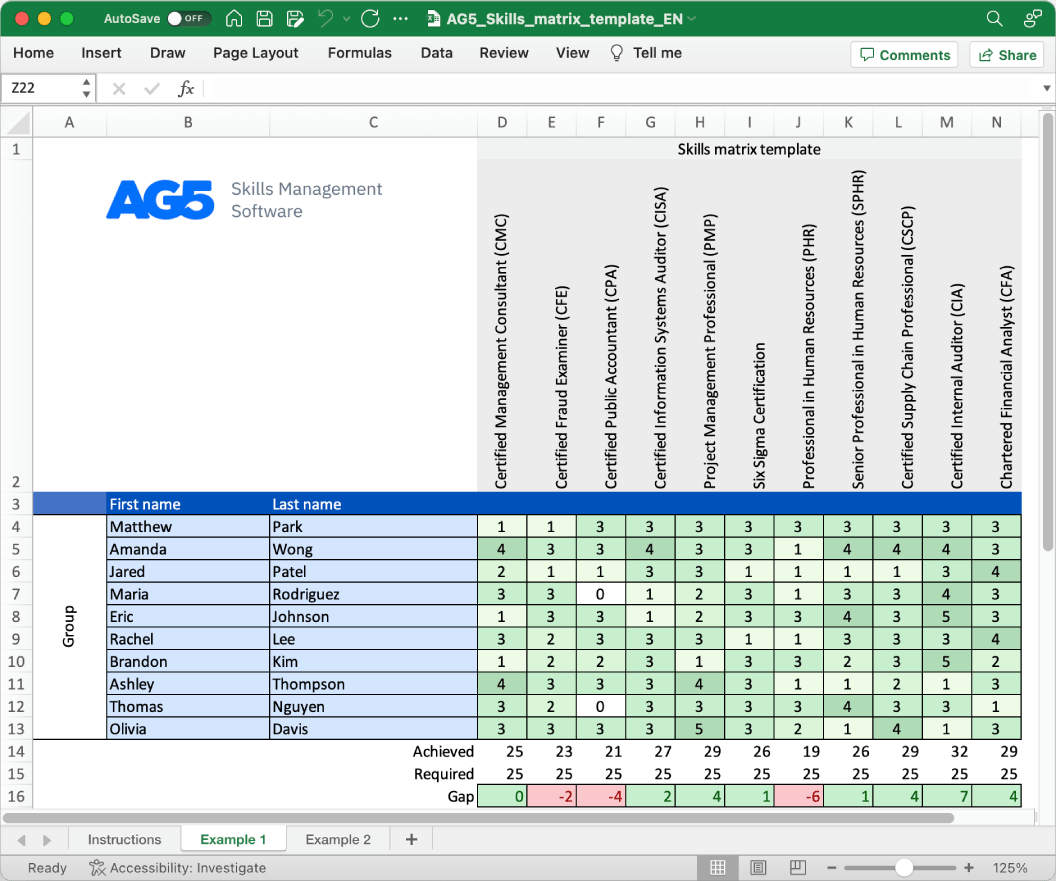
With our free QbD skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
- Certified Quality by Design Professional (CQbDP)
- Certified Pharmaceutical Quality by Design Specialist (CPQDS)
- Certified Quality by Design Practitioner (CQbDP)
- Certified QbD Lead (CQbDL)
- Certified QbD Facilitator (CQbDF)
- Certified QbD Specialist (CQbDS)
- Certified QbD Auditor (CQbDA)
- Certified QbD Consultant (CQbDC)
- Certified QbD Trainer (CQbDT)
- Certified QbD Expert (CQbDE)
- Certified QbD Process Engineer (CQbDPE)
- Certified QbD Project Manager (CQbDPM)
- Certified QbD Team Leader (CQbDTL)
- Certified QbD Implementation Specialist (CQbDIS)
- Certified QbD Risk Analyst (CQbDRA)
- Certified QbD Documentation Specialist (CQbDDS)
- Certified QbD Validation Engineer (CQbDVE)
- Certified QbD Product Development Professional (CQbDPDP)
- Certified QbD Regulatory Affairs Specialist (CQbDRAS)
- Certified QbD Performance Metrics Analyst (CQbDPMA)
Benefits Copied
Skills management software is important in QbD because it enables organizations to identify and assign personnel with the necessary skills and expertise required for implementing and maintaining quality-driven design processes, ensuring effective execution of QbD principles.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management