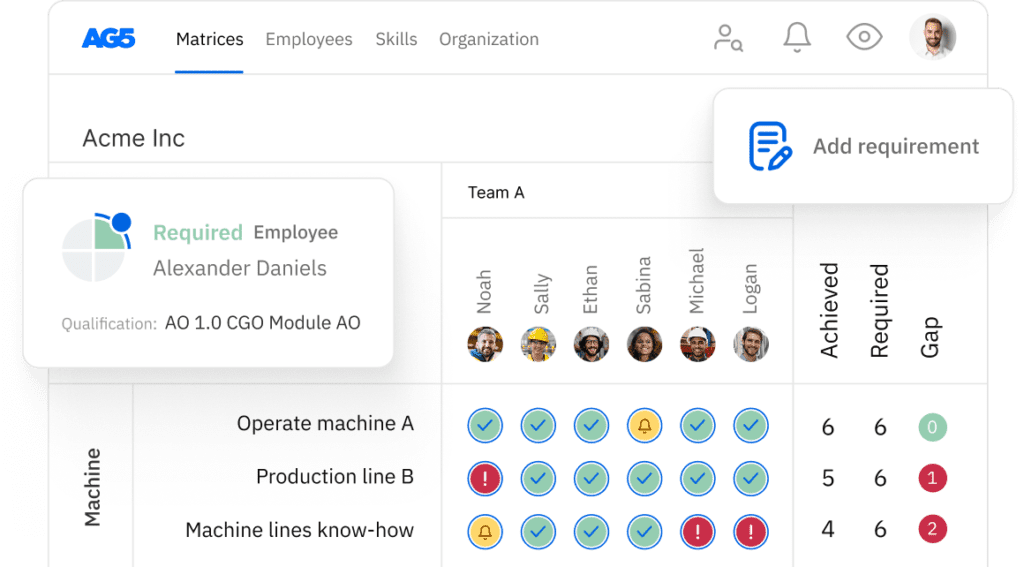
Pharmaceutical skills matrix template
A skills matrix template is a tool that can be used in the pharmaceutical industry to effectively manage and assess the skills and knowledge of individual employees or teams.
Download your free template here
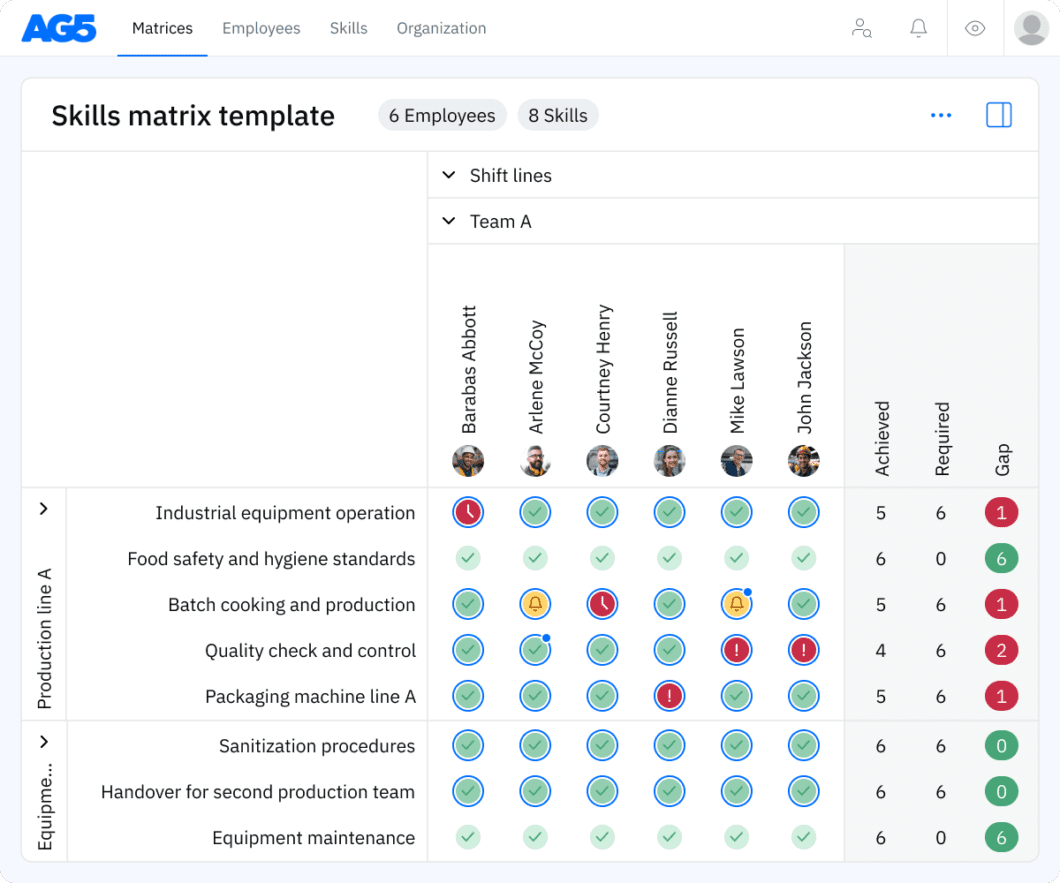
Overview Copied
A skills matrix template is a tool that organizations can use to effectively manage and assess the skills and certification statuses of individual employees or teams.
For example, you can use this pharmaceutical skills matrix to monitor the certification statuses of
Good manufacturing practices (GMP)
- Understanding and application of GMP guidelines in pharmaceutical manufacturing
- Conducting GMP audits and inspections for compliance
- Documenting manufacturing processes according to GMP standards
- Implementing corrective actions for GMP non-compliance issues
- Training staff on GMP requirements and best practices
Quality control and assurance
- Performing in-process and final product quality testing
- Conducting stability studies to ensure product shelf-life
- Implementing quality control procedures for raw materials and finished products
- Managing deviations, CAPAs (Corrective and Preventive Actions), and change controls
- Ensuring compliance with pharmacopeial standards (e.g., USP, EP)
Validation and qualification
- Developing and executing validation protocols (IQ, OQ, PQ) for equipment and processes
- Validating manufacturing processes to ensure consistent product quality
- Conducting cleaning validation to prevent cross-contamination
- Maintaining validation documentation and records for audits
- Revalidating processes and equipment based on changes or updates
Pharmaceutical manufacturing processes
- Operating and maintaining pharmaceutical manufacturing equipment (e.g., granulators, tablet presses)
- Knowledge of sterile manufacturing and aseptic processing techniques
- Implementing process optimization for improved yield and efficiency
- Managing batch production records (BPRs) and ensuring traceability
- Understanding and applying principles of continuous manufacturing in pharmaceuticals
Benefits Copied
Skills management software can help pharmaceutical organizations identify and track employee skills, streamline training and development, improve productivity, and ensure compliance in a highly regulated industry.
Download the free Excel Pharmaceutical skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management