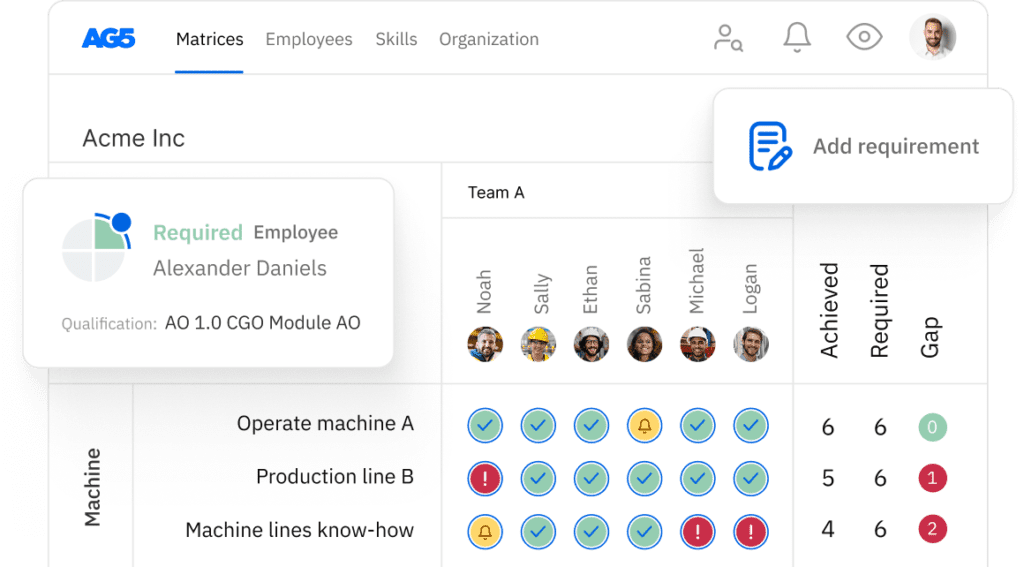
Good laboratory practices (GLP) skills matrix template
A skills matrix template is a tool GLP teams can use to effectively manage and assess their skills and knowledge.
Download your free template here
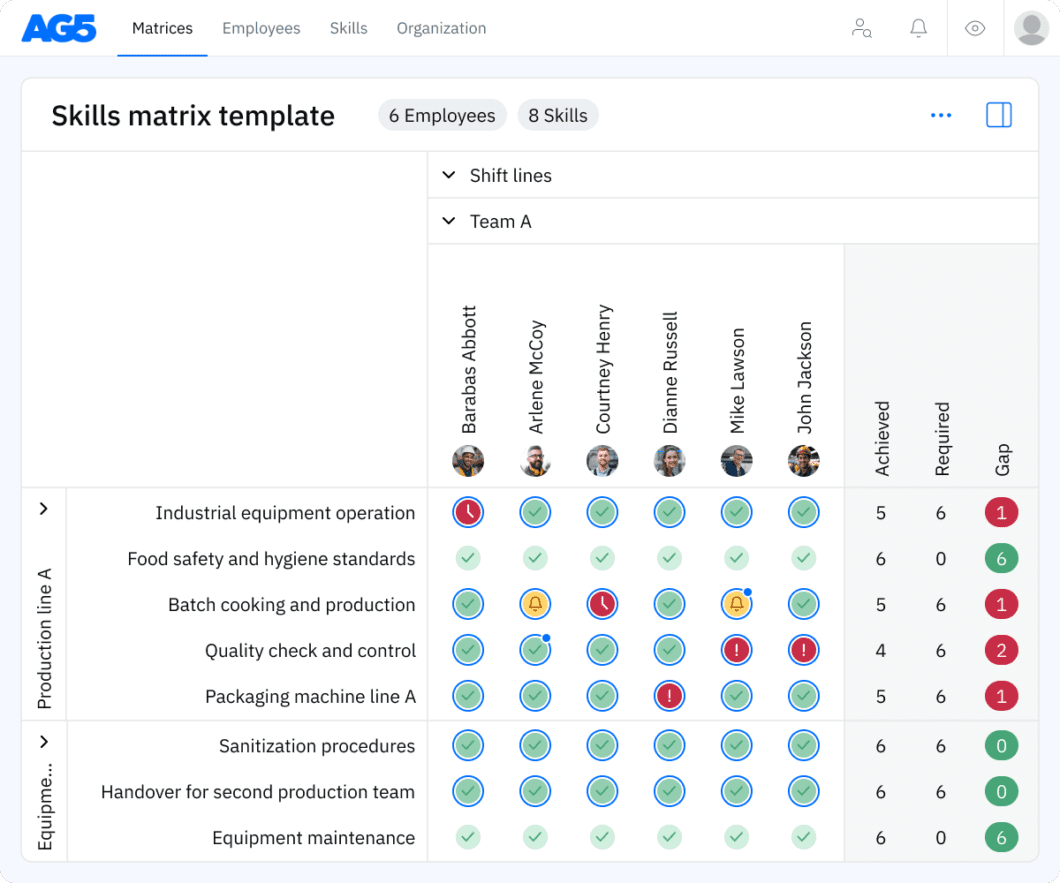
Overview Copied
With our free GLP skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
GLP compliance and regulatory affairs
- Certified Good Laboratory Practices Professional (CGLPP)
- Certified GLP Compliance Professional (GLPCP)
- Certified GLP Regulatory Affairs Professional
- Managing GLP compliance across laboratory operations
- Oversight of GLP deviations and corrective actions
- Certified GLP Compliance Officer
GLP auditing and quality assurance
- Certified GLP Auditor
- Certified GLP Quality Assurance Specialist
- Certified GLP Professional (GLPP)
- Developing and maintaining GLP quality assurance programs
- Implementing corrective and preventive actions (CAPA)
Study management and coordination
- Certified GLP Study Director
- Certified GLP Study Coordinator
- Certified GLP Analyst
- Managing GLP-compliant study designs and protocols
- Documenting study findings and ensuring adherence to protocols
Data integrity and documentation
- Certified GLP Documentation Specialist
- Certified GLP Data Integrity Specialist
- Accurate recording and management of GLP study data
- Implementation of data security and protection protocols
- Ensuring compliance with data retention and archiving requirements
- Reviewing data for completeness, accuracy, and integrity
- Monitoring electronic data capture systems for GLP compliance
Training and education
- Certified GLP Trainer
- Developing and updating GLP training materials
- Assessing the effectiveness of GLP training programs
- Ensuring ongoing professional development in GLP standards
- Monitoring competency and certification of laboratory personnel
Laboratory management and operations
- Certified GLP Facility Manager
- Certified GLP Quality Control Analyst
- Certified GLP Quality Management Representative
- Oversight of laboratory operations and compliance
- Managing cleanroom and controlled environment conditions
Analytical and scientific roles
- Certified GLP Biostatistician
- Certified GLP Analytical Chemist
- Certified GLP Toxicologist
- Certified GLP Pathologist
- Designing and executing GLP-compliant analytical studies
Benefits Copied
Skills management software assists in GLP compliance by tracking and managing employee skills and certifications related to laboratory practices. It ensures that laboratory personnel possess the required competencies to conduct accurate and reliable laboratory testing, maintain data integrity, and comply with GLP guidelines.
Download the free Excel Good laboratory practices (GLP) skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management