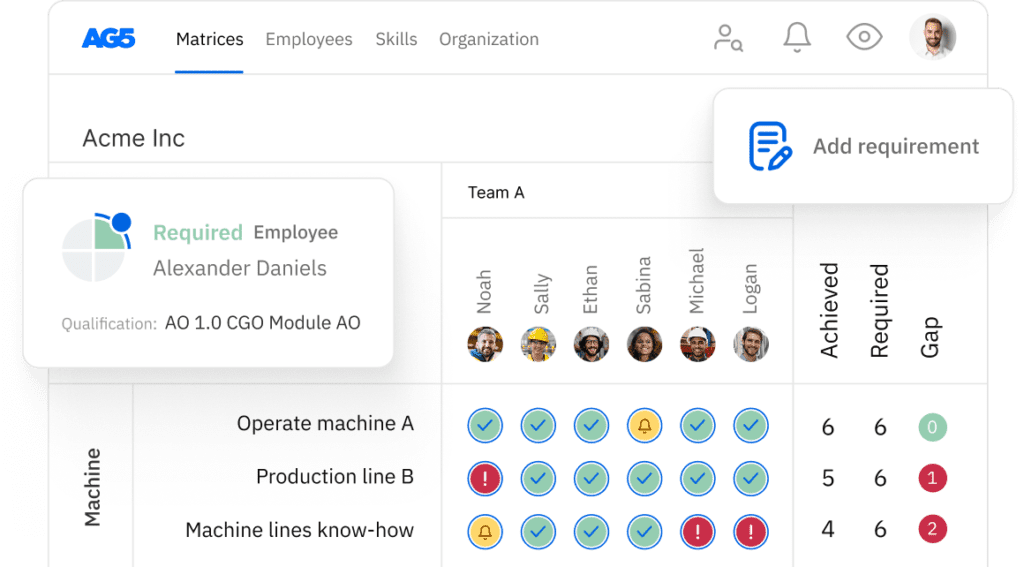
Good manufacturing practices (GMP) skills matrix template
A skills matrix template is a tool GMP teams can use to effectively manage and assess their skills and knowledge.
Download your free template here
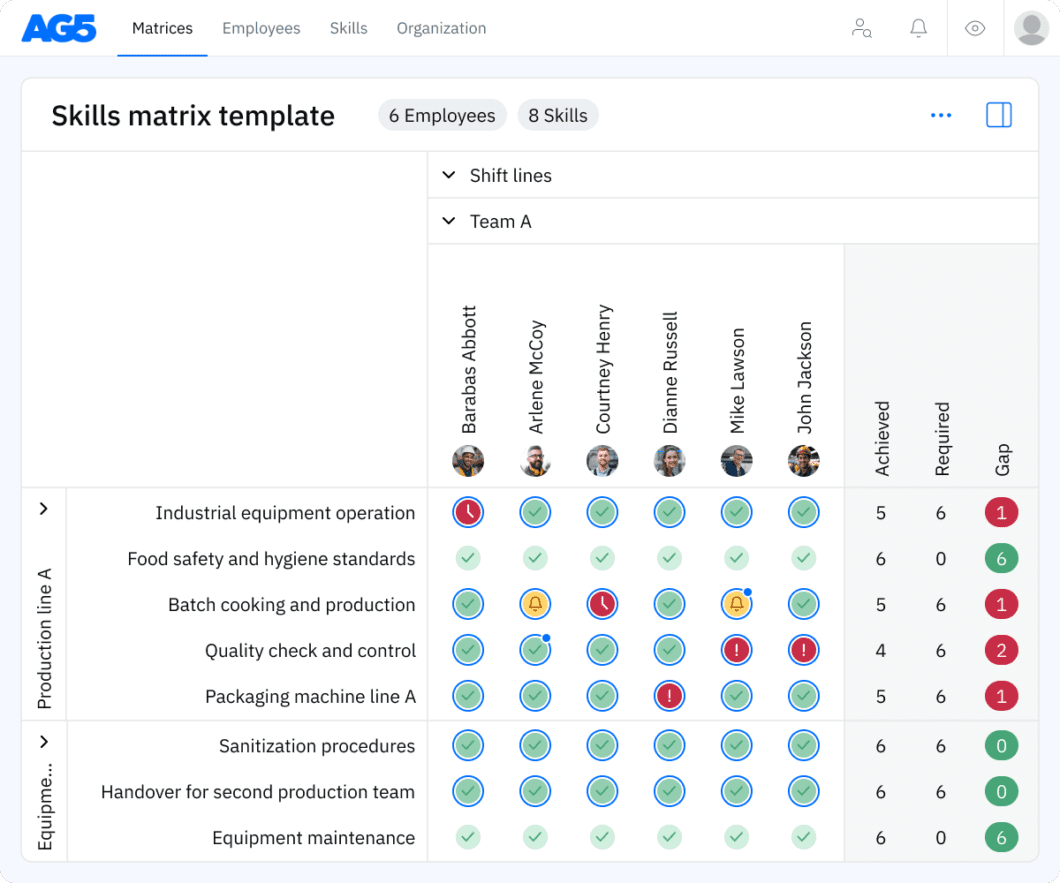
Overview Copied
A skills matrix template is a tool that organizations can use to effectively manage and assess the skills and certification statuses of individual employees or teams.
For example, you can use this free GMP skills matrix to track employees who are capable of leading an internal GMP audit, helping you ensure safety and compliancy ahead of an external audit.
GMP compliance and documentation
- Developing and maintaining GMP-compliant Standard Operating Procedures (SOPs)
- Ensuring accurate and timely documentation of manufacturing processes
- Conducting internal audits to verify GMP compliance
- Managing deviations and implementing corrective actions to maintain GMP standards
- Preparing for and managing external GMP inspections and audits
Quality control and assurance
- Implementing quality control tests for raw materials, intermediates, and finished products
- Monitoring production processes to ensure adherence to GMP standards
- Conducting product release testing and batch record review
- Identifying and addressing non-conformances in manufacturing processes
- Implementing CAPA (Corrective and Preventive Actions) based on quality findings
Facility and equipment management
- Conducting regular facility inspections to ensure GMP compliance
- Managing the qualification and validation of manufacturing equipment
- Implementing preventive maintenance programs for critical equipment
- Monitoring environmental controls in manufacturing areas (e.g., temperature, humidity)
- Documenting and reviewing facility and equipment maintenance activities
Raw material and supplier management
- Qualifying and auditing suppliers for GMP compliance
- Managing the receipt, storage, and handling of raw materials according to GMP
- Conducting incoming material inspections and testing for quality assurance
- Implementing traceability systems for raw materials throughout the manufacturing process
- Managing supplier non-conformances and implementing corrective actions
Benefits Copied
Using a skills matrix template can benefit your teams in various ways. Among them:
- Improved compliance and quality assurance. You can use this GMP skills matrix template to build teams with the necessary expertise required to adhere to stringent GMP regulations.
- Streamlined internal auditing and external audit preparation. Using this GMP skills matrix, you can easily track certifications and skills required for both internal and external audits and inspections.
- Succession planning. By using your GMP skills matrix to identify potential gaps in expertise due to turnover or promotions, you can proactively plan for succession, developing a pipeline of talent that will help ensure continuity in GMP compliance.
Download the free Excel Good manufacturing practices (GMP) skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
FAQs Copied
-
What are the benefits of using a GMP skills matrix?
-
Is this GMP skills matrix free to download?
-
How do I use a GMP skills matrix?
-
What if I want to take my skills management to the next level?
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management