ISO 13485: Medical Devices – Quality Management Systems
ISO 13485 is a standard that is specifically tailored to address the unique regulatory and quality management needs of the medical device industry. In this guide, we will explore the key aspects of ISO 13485, including its significance, steps required for certification, benefits of implementation, and more.

What is ISO 13485? Copied
ISO 13485 is an international standard that specifies requirements for a Quality Management System (QMS) for organizations involved in the design, development, production, installation, and servicing of medical devices.
Who needs to be certified in ISO 13485? Copied
Medical device manufacturers and organizations involved in the design, development, production, installation, and servicing of medical devices should seek certification in ISO 13485. While not mandatory, compliance with the standard demonstrates commitment to quality management and regulatory requirements in the medical device industry.
What is the ISO 13485 certification cost? Copied
The cost of ISO 13485 certification varies depending on factors such as the size of the organization, the scope of the certification, and the chosen certification body. Typically, it can range from a few thousand to tens of thousands of dollars. For an accurate estimate, it is best to contact a certification body directly.
The benefits of ISO 13485 certification Copied
Implementing ISO 13485 offers several benefits to organizations. Some key advantages include:
Regulatory compliance
ISO 13485 is harmonized with many global medical device regulations and requirements. By adopting the standard, organizations can align their quality management processes with regulatory expectations, making it easier to obtain regulatory approvals and market access for their medical devices.
Increased product quality and safety
ISO 13485 emphasizes risk management and quality throughout the entire medical device lifecycle. By following the standard’s requirements, organizations can identify and mitigate potential risks, leading to improved product quality, safety, and reliability.
Customer confidence
Certification in ISO 13485 demonstrates a commitment to producing safe and effective medical devices. This can enhance customer confidence in the organization’s products and services, leading to stronger relationships with customers, healthcare professionals, and other stakeholders in the medical device industry.
How to get certified in ISO 13485 Copied
To obtain ISO 13485 certification, an organization must develop and implement a QMS adhering to ISO 13485 requirements. Next, it should engage an accredited certification body for conducting an audit and addressing any non-conformities. Once the organization successfully passes the certification audit, it will receive ISO 13485 certification.
Challenges in implementing ISO 13485 Copied
Implementing ISO 13485 may pose several challenges to laboratories and organizations. They include:
Resource allocation
Developing and implementing a compliant QMS requires significant time, effort, and resources. Organizations may struggle to allocate the necessary personnel, funds, and expertise, especially for smaller companies or those with limited budgets.
Complexity of regulatory requirements
The medical device industry is subject to numerous and evolving regulatory requirements. Ensuring alignment between ISO 13485 and specific country or regional regulations can be challenging, requiring a deep understanding of both the standard and local regulatory frameworks.
Cultural change and training
Adopting ISO 13485 often requires a cultural shift within the organization, emphasizing a quality-focused mindset at all levels. Employees need to be trained and educated on the standard’s requirements, leading to changes in processes, documentation, and communication, which may encounter resistance or lack of awareness.
What are tips and strategies for preparing for ISO 13485? Copied
Here are a few tips for preparing for an ISO 13485 certification:
- Assess current processes against ISO 13485 requirements to identify areas for improvement
- Gain leadership support and commitment to foster a culture of quality
- Train staff on ISO 13485 principles, roles, and responsibilities
- Implement effective documentation practices for easy retrieval and version control
- Regularly review QMS effectiveness and compliance to identify and address issues
What are the renewal requirements for ISO 13485? Copied
The renewal requirements for ISO 13485 certification typically involve conducting a surveillance audit by the accredited certification body. This audit verifies the organization’s ongoing compliance with the standard’s requirements. ISO 13485 certifications are usually valid for a specific period, often three years, and need to be renewed to maintain the certification status.
What are resources for ISO 13485 certification? Copied
International Organization for Standardization (ISO). The ISO 13485 page on the official ISO website provides the ISO 13485 standard document, news updates, and additional resources.
Accredited certification bodies. To pursue ISO 13485 certification, you can reach out to accredited certification bodies that offer certification services in your region. These bodies have the expertise to guide you through the certification process. You can find a list of accredited certification bodies on the website of the International Accreditation Forum (IAF) or contact your local accreditation body.
International Electrotechnical Commission (IEC). The IEC collaborates with ISO to develop standards for electrical and electronic technologies. For medical devices with electrical components, IEC standards may be referenced in conjunction with ISO 13485.
Medical device regulatory authorities. Various regulatory authorities worldwide, such as the U.S. Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in the European Union, are closely related to ISO 13485. These regulatory bodies may use ISO 13485 as a reference or harmonize their regulations with the standard to assess medical device manufacturers’ compliance.
Free skills matrix template. AG5 offers a free industry skills matrix template for ISO 13485. Using it, you can develop and implement a plan to ensure that your employees’ ISO 13485-related skills are up to date, comprehensive, compliant, and ready for the future.
Skills management Copied
Skills management for ISO 13485
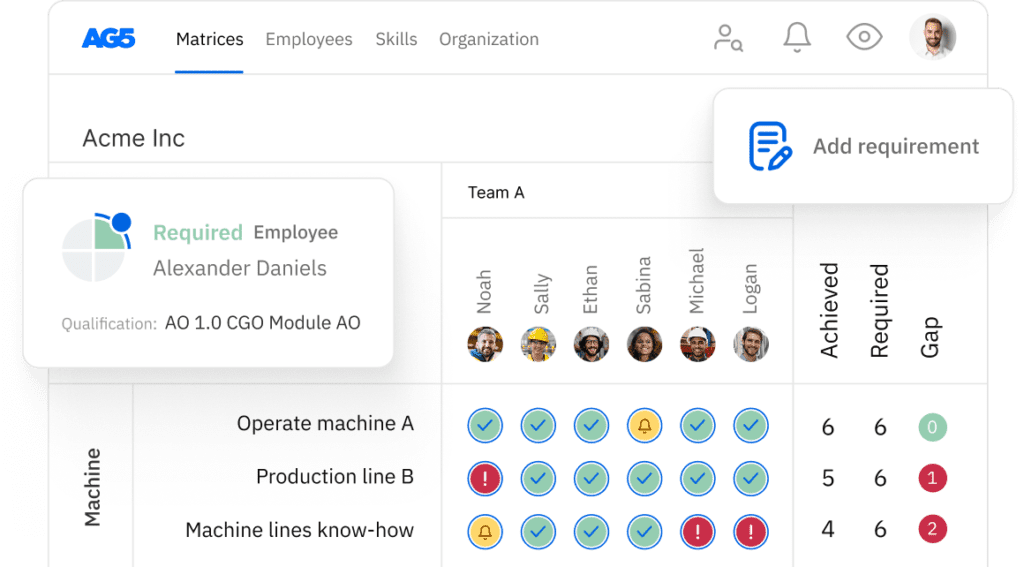
AG5 stores all certifications in the cloud, providing all authorized personnel with access to the right version of approved certifications. This helps you easily keep track of all data and documentation related to an ISO 13485 certification across your organization.
Using AG5’s skills management software, you can monitor the status of any type of certification that is relevant to your workforce, leveraging intuitive dashboards that provide you with a clear understanding of exactly what is needed to keep your employees skilled and safe.
FAQs about ISO 13485 Copied
-
What is the scope of ISO 13485?
-
Is ISO 13485 certification mandatory?
-
How long does it take to obtain ISO 13485 certification?
-
What are the cost considerations for ISO 13485 certification?
-
What is the validity period of ISO 13485 certification?
-
Can ISO 13485 be integrated with other management systems?
-
How can you learn more about ISO 13485 certification?
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management