GLP: Good Laboratory Practice
Good Laboratory Practice principles are a set of internationally recognized guidelines for non-clinical health and environmental studies. In this guide, we will explore the key aspects of GLP principles, including their significance, steps required for compliance, benefits of implementation, and more.

What is Good Laboratory Practice (GLP)? Copied
GLP principles were created by the Organization for Economic Cooperation and Development (OECD) for non-clinical trials conducted by manufacturers of certain products or medications [1].
Non-clinical trials focus on testing drugs or treatments in laboratory or animal settings, while clinical trials usually involve testing on human participants.
The purpose of GLP principles is to ensure the quality, reliability, traceability, and integrity of data in non-clinical studies. The principles encompass the following areas:
- Quality Management Systems for laboratory operations
- Standard Operating Procedures (SOPs) for laboratory activities and processes
- Training and qualification for employees
- Facilities and equipment maintenance, calibration, and validation
- Documentation and record-keeping for experimental procedures, observations, raw data, calculations, and results
- Data integrity and management of all study data (including data collection, storage, analysis, and reporting)
- Quality Assurance (QA) measures, including internal audits, inspections, and reviews
Who needs to comply with GLP? Copied
GLP apply to manufacturers that conduct non-clinical trials of products or medications in the following areas:
- Chemicals
- Pharmaceuticals
- Veterinary medicines
- Pesticides
- Biocides
Compliance with GLP is usually mandatory. However, these guidelines can vary from country to country, including the USA, UK, and EU, where there are may be specific regulations or interpretations of GLP principles.
How to earn a GLP certification Copied
GLP is not a certification, rather a set of principles with which organizations must comply [2]. To demonstrate compliance, organizations can undergo inspections or audits from regulatory agencies or accreditation bodies.
Individual employees can also participate in training programs that help them understand and implement GLP principles in the workplace.
Renewal requirements for GLP compliance Copied
Because GLP principles are not a certification, there are no renewal requirements. However, some organizations may be required to undergo audits to ensure that they maintain compliance.
The frequency of these audits depends on the type of organization and its geographical location. Organizations conducting nonclinical trials that fall under the GLP’s umbrella should check with their relevant regulatory agency, such as the FDA [3] or European Medicines Agency.
The benefits of implementing GLP Copied
Implementing GLP principles offers several benefits to organizations. Some key advantages include the following.
Regulatory compliance
Adhering to GLP principles is mandatory for manufacturing organizations that conduct certain types of nonclinical trials. This means that compliance with GLP principles is critical for staying operational.
Improved data and product quality
GLP compliance proves that an organization has standards in place to improve data reliability, consistency, and traceability. This not only improves process efficiency, but helps ensure a higher-quality final product.
Increased process efficiency
GLP principles helps organizations streamline internal processes – in terms of both data collection and operations – leading to more efficient processes that can result in time and cost savings.
Challenges in implementing GLP Copied
Implementing GLP principles may pose several challenges to organizations. They include the following.
Resource allocation
Implementing GLP principles encompasses training, infrastructure, and documentation. This can be resource-intensive, in terms of time and cost.
Cultural shift
Compliance with GLP principles means your organization will need to adapt. You will need a way to measure and track compliance on an individual, team, and departmental level, as well as a method of ensuring that compliance is continuous.
Complexity management
GLP compliance involves developing and maintaining a thorough understanding of all the framework’s principles. It also necessitates a method for maintaining up-to-date knowledge of evolving requirements.
Tips and strategies for preparing for GLP compliance Copied
Here are a few tips for preparing for a GLP compliance audit:
- Familiarize yourself with the OECD’s official GLP principles
- Review the European Commission’s “GLP Questions & Answers” document
- Read the FDA’s “Good Laboratory Practice (GLP) 101” document
Resources for GLP compliance Copied
For more information and guidance on GLP compliance, you can refer to the following resources.
Organization for Economic Cooperation and Development (OECD). The OECD’s website should be the first stop for organizations that must comply with GLP principles. There, you will find the principles themselves, as well as links to relevant websites and regulatory agencies.
European Commission. The European Commission’s website also offers a wealth of resources regarding GLP compliance for organizations operating throughout Europe. It also provides a list of all national bodies that monitor adherence to GLP principles, as well as a “Questions & Answers” document, which is linked above.
Food and Drug Administration (FDA). The FDA outlines GLP principles for organizations operating in the United States. It also provides links to the full principles required in the United States, the “GLP 101” document, and more.
ISO 9001 certification guide. AG5 offers a free certification guide for ISO 9001 – a standard organizations often use as a starting point when seeking to comply with GLP principles.
ISO 14001 certification guide. AG5’s free certification guide for ISO 14001 is also often integrated with GLP principles, and may prove useful to organizations.
Skills management for GLP principles Copied
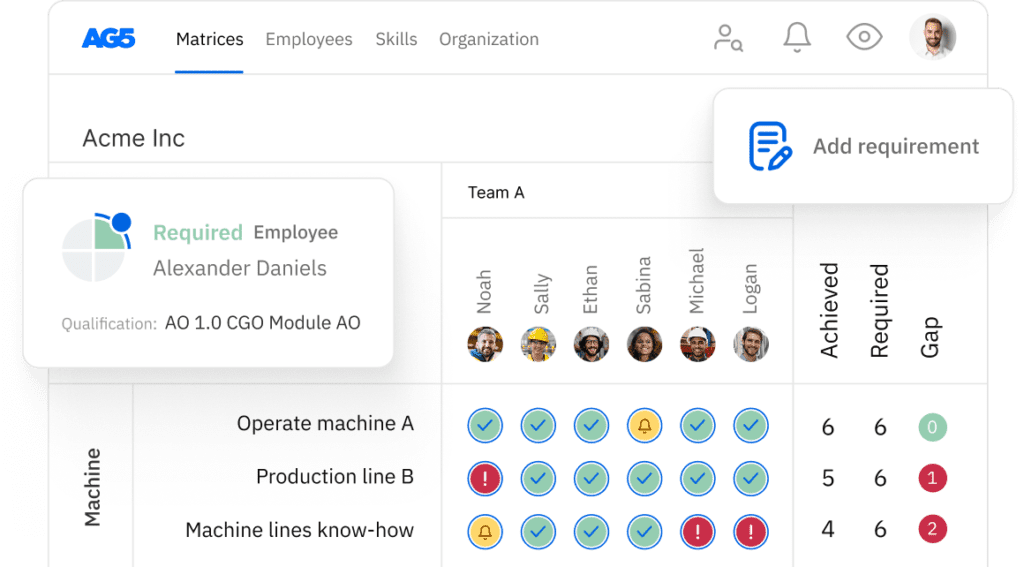
AG5 stores all certifications in the cloud, providing all authorized personnel with access to the right version of approved certifications. This helps you easily keep track of all data and documentation related to GLP compliance across your organization.
Using AG5’s skills management software, you can monitor the status of any type of certification that is relevant to your workforce, leveraging intuitive dashboards that provide you with a clear understanding of exactly what is needed to keep your employees skilled and safe.
Frequently asked questions about GLP principles Copied
-
What is the scope of GLP principles?
-
Is GLP compliance mandatory?
-
How long does it take to achieve GLP compliance?
-
What are the cost considerations for GLP compliance?
-
What is the validity period of GLP compliance?
-
Can GLP principles be integrated with any management systems?
-
How can you learn more about GLP compliance?
Sources Copied
- Change view: Table
-
APA
| # | Source title | Description | Publication | Retrieved | Source URL |
|---|---|---|---|---|---|
| 1 | OECD Principles of Good Laboratory Practice (GLP) and GLP Compliance Monitoring | OECD | - | May 24, 2024 | https://www.oecd.org/chemicals.. |
| 2 | Good Laboratory Practice | European Commission | - | May 24, 2024 | https://single-market-economy... |
| 3 | Nonclinical Laboratories Inspected under Good Laboratory Practices | FDA | - | May 24, 2024 | https://www.fda.gov/inspection.. |
Author Copied
Revisions Copied
Written by: Rick van Echtelt
Copy edited by: Adam Kohut
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management