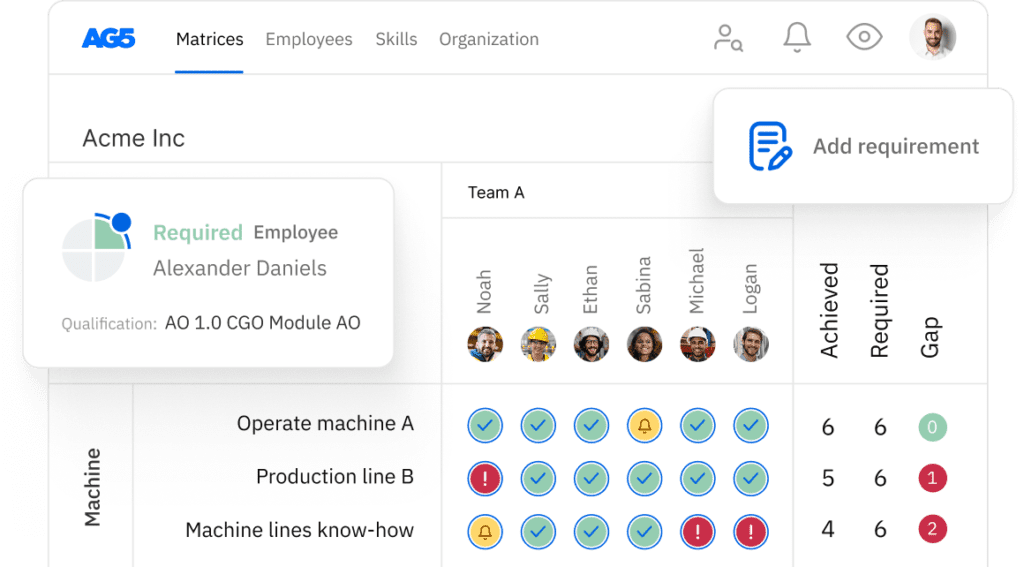
Process validation protocol execution skills matrix template
A skills matrix template is a tool teams can use to assess their process validation protocol execution skills and knowledge
Download your free template here
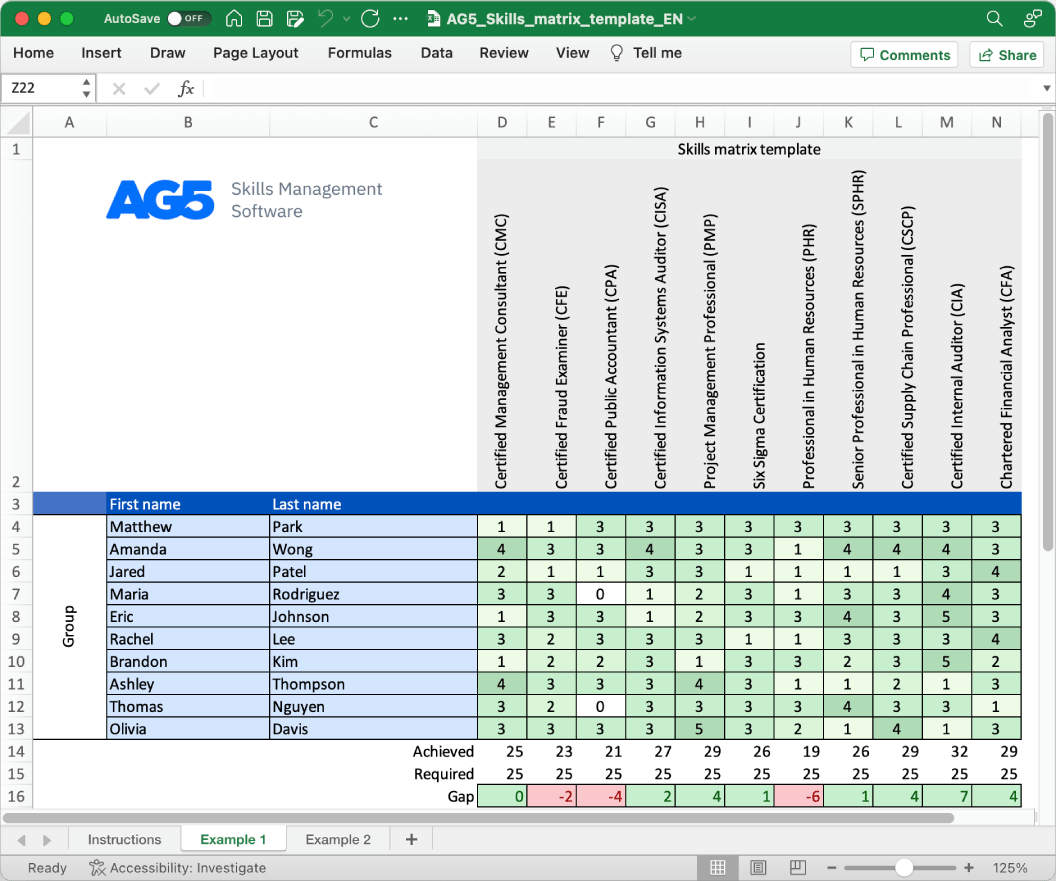
Overview Copied
With our free process validation protocol execution skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
- Certified Validation Professional (CVP)
- Certified Process Validation Professional (CPVP)
- Certified Process Validation Specialist (CPVS)
- Certified Process Validation Engineer (CPVE)
- Certified Process Validation Coordinator (CPVC)
- Certified Process Validation Manager (CPVM)
- Certified Process Validation Analyst (CPVA)
- Certified Process Validation Technician (CPVT)
- Certified Process Validation Expert (CPVE)
- Certified Process Validation Consultant (CPVC)
- Certified Process Validation Analyst (CPVA)
- Certified Process Validation Technician (CPVT)
- Certified Process Validation Expert (CPVE)
- Certified Process Validation Consultant (CPVC)
- Certified Process Validation Manager (CPVM)
- Certified Process Validation Analyst (CPVA)
- Certified Process Validation Technician (CPVT)
- Certified Process Validation Specialist (CPVS)
- Certified Process Validation Engineer (CPVE)
- Certified Process Validation Coordinator (CPVC)
Benefits Copied
Skills management software is important in process validation protocol execution, as it ensures successful execution by assigning qualified personnel with the necessary expertise for each validation step.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management