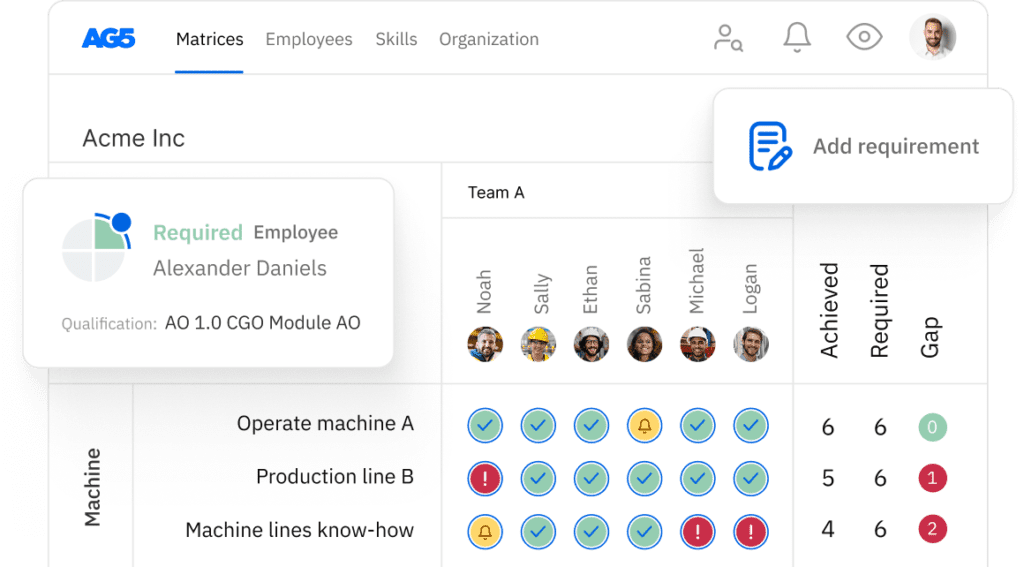
Clean-in-place (CIP) system skills matrix template
A skills matrix template is a tool teams can use to assess their CIP system skills and knowledge
Download your free template here
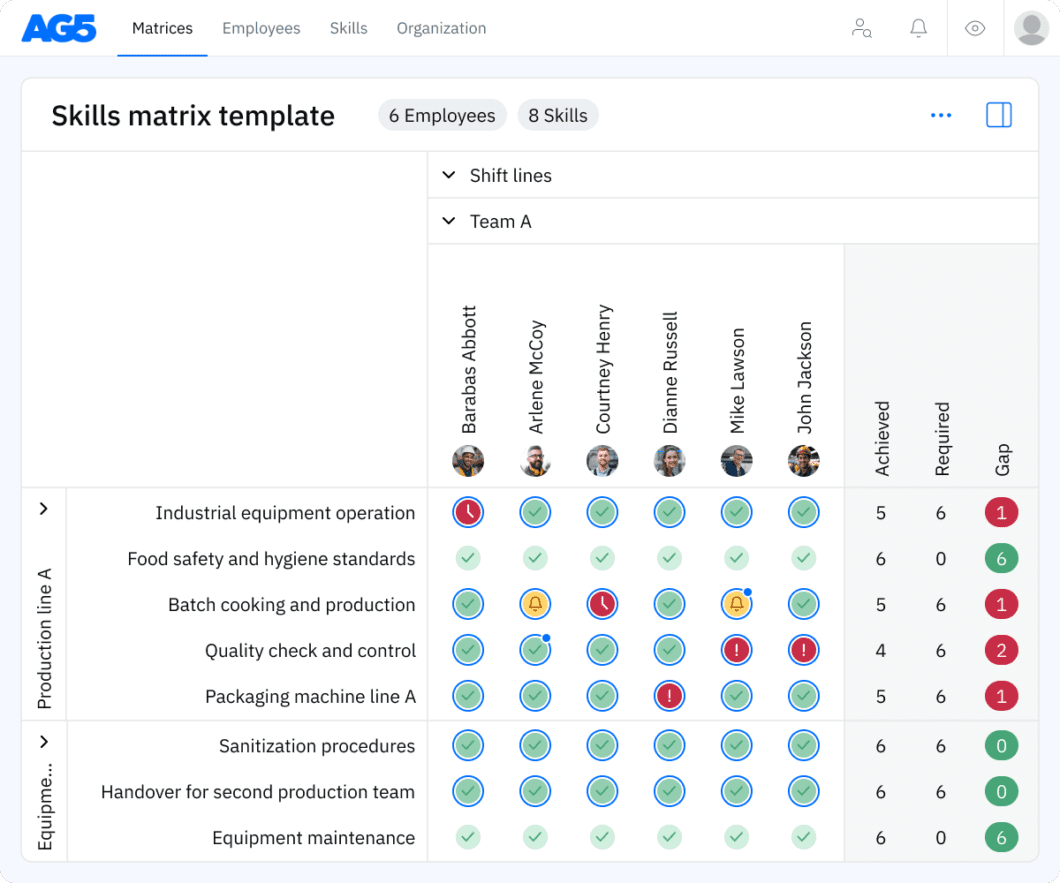
Overview Copied
With our free CIP system skills matrix template, you will receive a clear overview of the skills that are present in your organization, as well as those that are missing. Using this information, you can develop and implement a plan to ensure that your employees’ skills are up to date, comprehensive, compliant, and ready for the future.
CIP system operation
- Understanding CIP system components and their functions
- Operating automated and manual CIP systems
- Setting up and configuring CIP cycles based on product requirements
- Monitoring and adjusting CIP parameters during operation
- Troubleshooting CIP system issues during operation
CIP process validation
- Developing and executing CIP validation protocols
- Conducting swab tests and microbial analysis for CIP validation
- Documenting and reviewing CIP validation results
- Ensuring compliance with industry standards (e.g., FDA, ISO) during validation
- Revalidating CIP processes after system modifications or maintenance
Chemical handling and safety
- Selecting appropriate cleaning agents and sanitizers for CIP processes
- Understanding the chemical properties and interactions of CIP cleaning agents
- Handling and storing CIP chemicals safely
- Implementing personal protective equipment (PPE) protocols for chemical handling
- Conducting safety training for employees on CIP chemical handling
System maintenance and calibration
- Performing routine maintenance on CIP system components (e.g., pumps, valves)
- Calibrating sensors and instruments used in CIP systems
- Managing and replacing CIP system filters and membranes
- Conducting preventive maintenance to avoid system downtime
- Documenting maintenance activities and ensuring system readiness
Benefits Copied
Skills management software is important in CIP systems to efficiently assign qualified personnel, track their expertise in CIP system design, chemical compatibility, monitoring and control, CIP system validation, and regulatory compliance, ensuring effective and reliable cleaning processes.
Download the free Excel Clean-in-place (CIP) system skills matrix template Copied
We also have a free Excel template available that you can download if you are not ready to get started with AG5. To download it, please complete this form here.
Author Copied
Revisions Copied
Tired of managing skills in Excel?
Say goodbye to Excel matrices. Start using AG5’s plug and play skill matrix software.
Recognized by G2 for Excellence in Skills Management