Medical device manufacturing software for skills, training, and audit readiness
Medical manufacturing requires precise control of workforce skills, training, and certifications. This guide explains medical skills management software, FDA and ISO 13485 requirements, why spreadsheets fail, and how audit-ready systems help teams track qualifications and reduce compliance risk.
Medical device manufacturing demands precision at every level. From the production floor to quality assurance, each task requires specific skills, up-to-date training, and clear documentation. As a result, managing employee qualifications across hundreds or thousands of tasks has become one of the biggest operational challenges in the industry.
For companies producing medical devices, medical equipment, or medical supplies, the stakes are particularly high. FDA inspections, ISO 13485 audits, and daily production demands all hinge on one critical question: Can you prove the right person, with the right skills, performed the right task?
This is where medical skills management software becomes essential. Rather than replacing your QMS or MES, these tools work alongside them to track who can do what, identify training gaps, and generate audit-ready reports in minutes instead of days.
In this guide, we’ll explore how medical manufacturing teams use software to manage workforce skills and what to look for when choosing the right system.
Why medical manufacturing needs dedicated skills managementCopied
Medical manufacturing isn’t like other industries. The regulatory environment is stricter, the margin for error is smaller, and the documentation requirements are more extensive. Therefore, the traditional approach of tracking employee qualifications in spreadsheets quickly breaks down.
The complexity of task-level requirements
In a typical medical device manufacturing facility, production involves hundreds or even thousands of distinct tasks. Each task may require:
- Specific technical skills (operating machinery, handling materials, performing quality checks)
- Current certifications (MedAccred, equipment operation, safety training)
- Compliance with Good Manufacturing Practice (GMP) standards
- Documented training on standard operating procedures (SOPs)
Meanwhile, your workforce is constantly evolving. Employees move between shifts, take on new responsibilities, complete training programs, and renew certifications. Tracking all of this in spreadsheets means you’re always working with outdated information. By the time you update one sheet, something else has changed.
As a result, production managers often can’t answer basic questions like ‘Who on the afternoon shift is qualified to run this equipment?’ without spending hours digging through files. This isn’t just inefficient – it’s a compliance risk.
FDA and ISO 13485 requirements for personnel qualification
FDA quality system requirements for device manufacturers are defined under 21 CFR 820 (QS Regulation), and FDA has issued a final rule (QMSR) to align more closely with ISO 13485. Both frameworks emphasize personnel qualification and training documentation.
During an FDA inspection or ISO 13485 audit, inspectors will ask to see evidence that:
- Employees performing specialized tasks have the necessary education, training, and experience
- Training records are complete, current, and accessible
- Your organization has a system for identifying training needs and addressing gaps
- Changes to personnel qualifications are documented and controlled
If you can’t produce this documentation quickly and accurately, you risk audit findings, warning letters, or production delays. This is why medical device manufacturing software focused on skills and training has become so important. It provides the structure and traceability that regulators expect.
What medical skills management software actually doesCopied
Medical skills management software isn’t about replacing your existing systems. Instead, it connects your workforce data to your production requirements in a trackable, auditable way.
Let’s break down the core functions that make this type of software valuable for medical manufacturing teams.
Mapping skills to specific production tasks
The foundation of effective skills management is understanding exactly which skills are needed for each task. This goes beyond job titles or general role descriptions. You need task-level granularity.
For example, ‘Production Technician’ might be a job title, but within that role, there could be dozens of distinct tasks:
- Operating specific manufacturing equipment
- Performing visual inspections
- Packaging finished products
- Documenting batch records
- Handling non-conforming materials
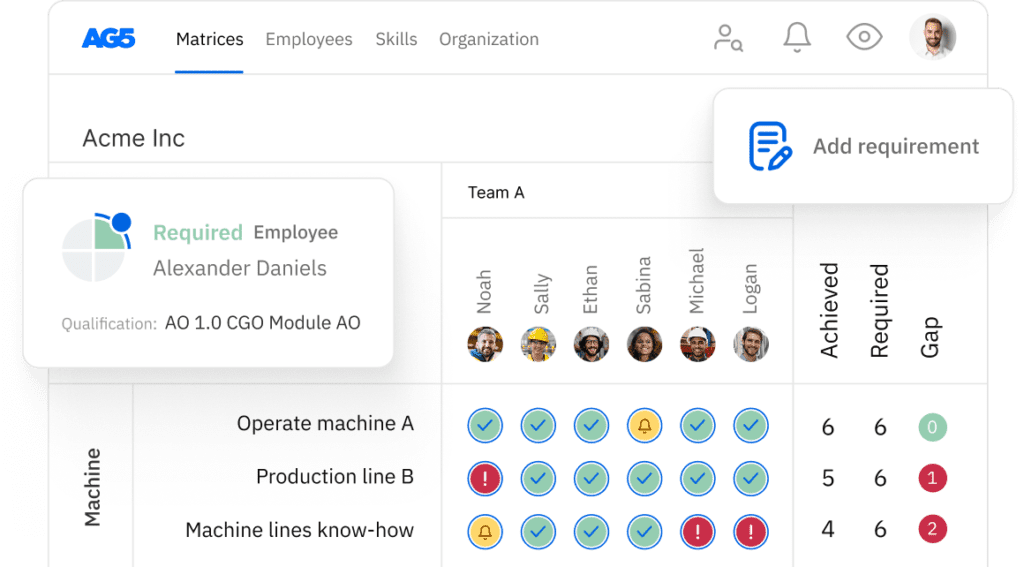
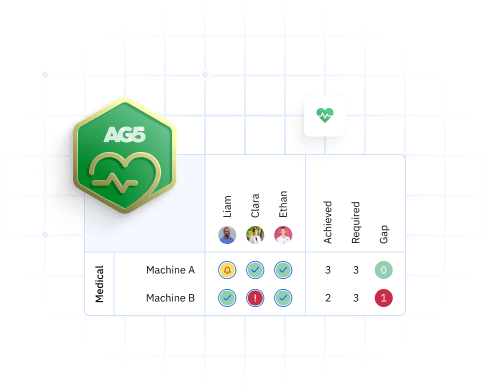
Each of these tasks requires different skills and qualifications. A skills matrix helps you map these relationships clearly. It shows you:
- Which employees can perform which tasks
- Where skill gaps exist across your workforce
- Who needs training to maintain coverage
- Whether you have backup coverage for critical tasks
This level of visibility is impossible to maintain in spreadsheets, but it’s essential for medical device manufacturing where every task must be performed by qualified personnel.
Tracking certifications and training expiration dates
Medical equipment manufacturing software needs to handle the reality that qualifications expire. Certifications need renewal. Training becomes outdated when procedures change. As a result, tracking expiration dates and sending timely alerts is critical.
Modern skills management platforms provide automatic notifications when:
- A certification is approaching expiration
- Training needs to be refreshed based on your internal policies
- An employee’s qualification for a critical task is about to lapse
- New requirements are added that affect existing workforce qualifications
These alerts help you stay ahead of problems. Instead of discovering during an audit that three key operators let their certifications expire, you get advance warning and can schedule renewals proactively.
For medical supplies manufacturing software applications, this feature prevents the common scenario where production gets delayed because qualified operators aren’t available for critical shifts.
Generating audit-ready reports
When an FDA inspector or ISO auditor asks to see your personnel qualification records, you need to produce complete, accurate documentation immediately. Scrambling to compile information from multiple spreadsheets, training systems, and HR databases isn’t acceptable.
Medical skills management software provides report generation that:
- Shows all qualifications for a specific employee
- Lists all qualified personnel for a specific task or process
- Documents training history with completion dates and instructors
- Tracks changes to qualifications over time
- Provides evidence that training needs were identified and addressed
This reporting capability transforms audit preparation from a week-long scramble into a few clicks. You can demonstrate compliance with personnel qualification requirements without disrupting operations or pulling team members off their regular work.
How medical manufacturing teams use skills management software dailyCopied
The real value of any software shows up in day-to-day operations, not just during audits. Here’s how medical manufacturing teams actually use skills management tools to solve practical problems.
Production planning and shift coverage
Production managers need to know: Can I staff tomorrow’s shift with qualified operators? Do I have backup coverage if someone calls in sick?
With medical device manufacturing software that includes workforce skills tracking, managers can:
- Filter available employees by the specific skills needed for upcoming production runs
- Identify cross-training opportunities to build more flexibility into their workforce
- Spot single points of failure where only one person can perform a critical task
- Make staffing decisions based on current qualifications rather than assumptions
This prevents the common problem where someone is assigned to a task they’re not actually qualified to perform – a situation that creates both compliance risks and quality issues.
Training needs analysis and resource allocation
Training programs are expensive and time-consuming. You can’t afford to train everyone on everything, but you can’t afford to have gaps either. Therefore, you need clear visibility into where training will have the most impact.
A competency management system helps you prioritize training investments by showing:
- Which skills gaps affect the most critical production tasks
- Where adding one trained person could provide backup coverage for multiple tasks
- Which teams or shifts have the biggest skill deficits
- How training completion rates compare across different departments
This data-driven approach to training planning ensures that your training budget goes toward building the capabilities that matter most for your operations.
Change control and document management
As procedures change, affected personnel must be retrained. When new equipment is introduced, you need clear visibility into who is qualified to operate it. And as regulations evolve, you must be able to prove your workforce has been trained on the updates.
Medical equipment manufacturing software with proper change control features provides:
- Audit trails that timestamp and document all changes to personnel qualifications
- Multi-person approval workflows for confirming and updating qualification records
- Version control for training materials and procedures
- Two-factor authentication for critical data updates to strengthen access control
These features ensure that your qualification records meet the same rigor and traceability standards as your other quality system documentation.
Choosing the right medical manufacturing skills management solutionCopied
Not all skills management software is created equal, especially when it comes to medical manufacturing. Here’s what to look for when evaluating solutions.
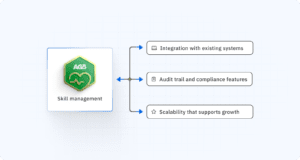
Integration with existing systems
Your skills management software shouldn’t exist in isolation. It needs to connect with your other business systems to be truly effective.
Look for solutions that offer integrations with:
- HR information systems (HRIS) for employee data synchronization
- Learning management systems (LMS) for training completion tracking
- Quality management systems (QMS) for documentation workflows
- Enterprise resource planning (ERP) systems for workforce planning
- Manufacturing execution systems (MES) for production task assignment
Integration capabilities prevent duplicate data entry, reduce errors, and ensure that qualification information stays current across all your systems.
Audit trail and compliance features
For medical device manufacturing, having a complete audit trail isn’t optional – it’s a regulatory requirement. Your skills management software needs to automatically document:
- Who made changes to qualification records
- When changes were made
- What the previous value was
- Why changes were made (through required comments or justification fields)
- Who approved changes (for systems with approval workflows)
This level of traceability demonstrates that your qualification records are controlled and trustworthy – exactly what auditors want to see.
Scalability for growth
Your operations will change over time. New products will be added. New facilities might open. Regulatory requirements will evolve. Therefore, your skills management solution needs to grow with you.
Consider whether the software can:
- Handle increasing numbers of employees, skills, and tasks without performance degradation
- Support multiple facilities or business units within a single system
- Accommodate custom fields and workflows specific to your operations
- Adapt to changing regulatory requirements without requiring complete system overhauls
- Provide role-based access control that supports complex organizational structures
Choosing a system that can scale prevents the need for costly replacements down the road.
Common mistakes to avoid when implementing skills management softwareCopied
Implementation success depends on avoiding these common pitfalls that can derail even the best software solutions.
Trying to migrate everything at once
Many teams make the mistake of trying to move all historical training records, certifications, and qualification data into a new system on day one. This creates an overwhelming data entry burden and delays the actual value delivery.
A better approach:
- Start with current, active employees and their critical qualifications
- Focus on skills that need frequent tracking and verification
- Keep historical records in your old system for reference during audits
- Add historical data gradually as needed rather than all at once
This phased approach gets you up and running faster while reducing implementation stress.
Making the skills library too complex
It’s tempting to create a comprehensive skills library that captures every possible nuance of your operations. However, too much complexity makes the system difficult to maintain and reduces user adoption.
Instead:
- Start with high-level skill categories that map to actual production tasks
- Add detail only where it affects qualification decisions or regulatory requirements
- Regularly review and consolidate similar skills to prevent duplication
- Focus on skills that can be objectively verified rather than subjective traits
Remember: the goal is practical workforce management, not creating a perfect theoretical model.
Neglecting change management and user training
Even the best medical skills management software will fail if your team doesn’t understand how it works or why it matters. Too many implementations focus solely on the technical setup while ignoring the people side.
Successful implementations include:
- Clear communication about why the change is happening and what problems it solves
- Role-specific training that shows users exactly how the system helps their daily work
- Champions in each department who can answer questions and provide peer support
- Quick reference guides and job aids for common tasks
- Regular feedback sessions to identify and address usability issues
When people understand the value and know how to use the system, adoption follows naturally.
The real-world impact of effective skills managementCopied
Let’s look at how medical manufacturing companies benefit from proper skills management software in practical, measurable ways.
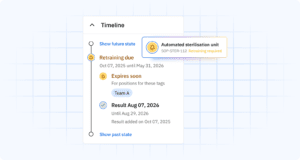
Faster audit preparation and response
Traditional audit preparation often takes a full week or more of dedicated effort. Staff members must pull records from multiple systems, verify their accuracy, compile them into presentable formats, and prepare explanations for any gaps.
With proper medical device manufacturing software for skills tracking, this process shrinks to hours instead of days. When an auditor asks ‘Show me the qualification records for everyone who worked on batch 12345,’ you can generate that report immediately.
As a result, audits become less disruptive to operations, and your team can focus on demonstrating compliance rather than scrambling to document it.
Reduced production delays from staffing issues
Production delays due to qualified personnel shortages cost medical manufacturing companies millions annually. Someone calls in sick, and suddenly you realize they were the only person qualified for a critical task. A new product launch gets delayed because training wasn’t completed on time.
Medical equipment manufacturing software that tracks skills and qualifications helps prevent these scenarios by:
- Identifying single points of failure before they cause problems
- Alerting managers to upcoming certification expirations that could affect production
- Enabling proactive cross-training to build backup coverage
- Providing clear visibility into who can substitute for whom in various production roles
Companies report that reducing unplanned production delays alone often justifies the investment in skills management software.
Better training ROI through targeted development
Training programs represent a significant investment. Every hour spent in training is an hour not spent on production. Therefore, you need to ensure that training dollars go toward building capabilities that actually matter for your operations.
Skills management software provides the data you need to:
- Identify which skills have the biggest coverage gaps
- Determine which employees are best positioned to learn new skills based on their current qualifications
- Track which training programs deliver the best results in terms of performance and retention
- Measure the impact of training investments on operational metrics like quality and productivity
- Demonstrate the value of training investments to leadership with concrete data
This data-driven approach to training ensures that your development programs actually build the capabilities your organization needs.
Moving forward with skills management softwareCopied
Medical manufacturing is becoming more complex. Regulatory requirements continue to tighten. Product portfolios expand. Workforce turnover creates ongoing training needs. Meanwhile, expectations for rapid audit response and flawless documentation only increase.
The companies that manage these challenges most effectively have moved beyond spreadsheets to purpose-built medical skills management software. They recognize that workforce qualification is a strategic capability affecting every aspect of operations.
Whether you’re producing medical devices, medical equipment, or medical supplies, the fundamentals remain the same:
- Map skills to specific production tasks so you know exactly who can do what
- Track certifications and training with automated alerts for upcoming expirations
- Generate audit-ready reports that demonstrate compliance in minutes, not days
- Integrate with your existing QMS, MES, and ERP systems to keep data synchronized
- Maintain complete audit trails that support FDA and ISO 13485 requirements
The question isn’t whether you need better visibility into workforce qualifications. The question is whether you’re ready to implement a system built specifically for medical manufacturing teams.
For teams ready to move beyond spreadsheets, platforms like AG5 provide the structure, traceability, and reporting capabilities that medical manufacturing demands. The software handles the complexity of tracking who can do what, identifies training gaps before they become problems, and generates the documentation you need to demonstrate compliance with FDA quality system requirements.
If you’re spending more time documenting qualifications than developing them, if audit preparation feels like an archaeological dig through old files, or if you can’t quickly answer basic questions about workforce coverage – it’s time to evaluate dedicated skills management software for your medical manufacturing operation.
FAQs Copied
-
Is AG5 medical device manufacturing software?
-
What is medical skills management software?
-
Does AG5 help with ISO 13485 readiness?
-
How does AG5 fit with QMS, MES, and ERP systems?
Author Copied
Revisions Copied
Written by: Rick van Echtelt
Copy edited by: Adam Kohut